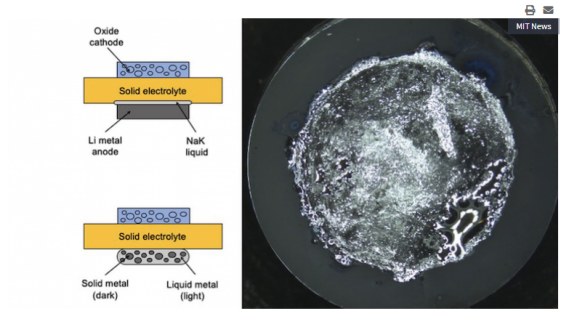
Placing a layer of liquid metal between the lithium anode and the solid electrolyte shows improvement in solid-state battery charging.
Anyone who has followed the development of solid-state lithium metal batteries knows that there are many challenges to be encountered during the charging process. The anode (negative electrode) in these batteries consists of a thin layer of lithium metal foil that provides much more lithium ions than the graphite anode used in lithium-ion batteries, and therefore produces a higher energy display density. During the charging process, lithium needle-like dendrites cover the lithium metal surface. The spreading of these needle-like dendritic lithium dendrites can cause the battery to short-circuit and cause a fire.
solid state battery
The solid-state portion of the battery comes from solid polymer or ceramic electrolyte materials that inhibit lithium needle dendrite diffusion. This has been partially successful, but dendrite growth continues to cause long-term design problems. The future of electric vehicles (EVs) is one that requires fast DC charging. Unfortunately, dendrite growth grows rapidly with faster charging, and so far this problem has largely prevented the large-scale commercialization of practical solid-state lithium batteries.
Researchers are trying to solve the problem in terms of improving battery performance. A team of scientists from the Massachusetts Institute of Technology (MIT), Texas A&M University (TAMU), Brown University, and Carnegie Mellon University (CMU) recently studied the use of a thin film of liquid metal electrode material on the surface of lithium metal anodes.
Liquid Metal Electrodes
The team was inspired to use melted metal electrodes in their experiments with high-temperature batteries. These batteries operate at several hundred degrees, so they are not suitable for use in portable battery-powered devices or electric cars. However, these batteries can be charged at high currents without the formation of harmful dendrites, MIT said in a press release, "The motivation for developing the electrodes was based on carefully selected alloy electrodes that were designed to act as self-healing components of the metal electrodes during the liquid phase of introduction."
To provide a similar liquid-solid interface, the team developed a semi-solid electrode placed between the lithium metal anode and the solid electrolyte material. This semi-solid electrode provides a surface layer that can self-heal, while also helping to prevent tiny cracks from appearing on the brittle electrolyte surface, which can provide a place for dendrite growth. The material of the semi-solid electrode is composed of sodium and potassium, similar to the solid metal mixtures used by dentists to fill cavities, but still able to flow and take shape.
According to news sources, the team was able to run the system on a liquid-metal interface that can use up to 20 times more current than solid lithium metal without forming any dendritic lithium dendrites.
The research team believes this new method can be adapted to different versions and structures of solid-state lithium batteries. One of the researchers, a CMU mechanical engineering professor, said, "We think we can use this method for any solid-state lithium-ion battery. The method can be used immediately for battery development, expanding the development of applications from handheld devices to electric vehicles to electric aviation."
It's too early to tell if the liquid metal layer between the lithium metal anode and the solid electrolyte is a breakthrough or just another step toward solid-state lithium batteries. By focusing on the properties of the metal electrode, rather than just the properties of the solid electrolyte, this research may inspire further innovation.
(Source: Zhongshun Xinneng Technology Department September 6, 2022 Responsibility: Wen Gong)
Reprinted for the purpose of transmitting more information, and does not mean that we agree with its views and is responsible for its authenticity.